Назад
Назад
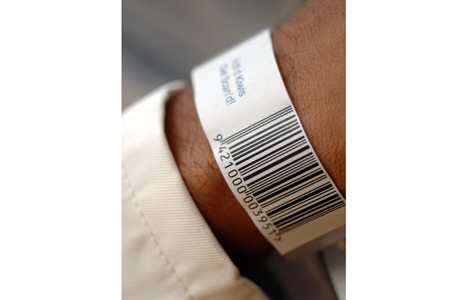
Solutions based on the GS1 standards ensure greater safety for patients in chemotherapy
The use of GS1 barcodes helps improve patient safety across the EU
Treatment of patients with cancer requires special precautionary measures to be taken when handling the respective medications. Special attention in hospital logistics is paid to the preparation, transport, storage, or disposal of cytostatics. Uniform GS1 standards, which have been successfully implemented in many sectors such as the food industry for many years, have recently been adopted by the Vienna General Hospital (AKH) as an aid in handling cytostatics.
In order to cut back on manual interventions and minimize the chance of errors along the supply chain, as well as to ensure greater traceability in the preparation of cytostatics, the AKH dispensary, CATO Software Solutions, GS1 Austria Healthcare, and key pharmaceutical manufacturers launched a joint project.
Mag. Elfriede Dolinar, project leader for the AKH dispensary, gives an insight into the process: "68 drugs are used in the preparation of cytostatics at the AKH, then the finished products are supplied to the AKH wards, the St. Anna Children's Hospital, and the Hera Sanatorium. The 68 drugs need to be logged in the SAP inventory control system as well as in the "CATO" in-house cytostatic preparation software system.”
The goal was to track the entire supply channel automatically by means of a scanner-based logistics system. Mag. Barbara Dorner, Business Development Manager Healthcare at GS1 Austria, gives an insight into the project: "GS1 standards uniquely identify products or delivery units and ensure continuous electronic traceability throughout all internal process flows. From delivery to the dispensary, arrival in the clean room, preparation and delivery to the ward, and administration at the hospital bed, the data on the products are automatically recorded and processed." The data are automatically recorded and processed with scanners. "We are thus able to verify the correctness of the information quickly and easily at various identification points and streamline the entire data management process," explains Dorner. The GS1 DataMatrix and the GS1-128 are read on the medication label, which contains an encoded GTIN (Global Trade Item Number) and a serial number.
According to Dorner, "The biggest challenge was the diversity of the data that had to be recorded: linear barcodes on registered drugs, barcodes on the secondary packaging, the primary packaging with the product name, expiration date, and batch number, as well as the information in plain text."
The GS1 approach to a solution was carried out in two subprojects. Step 1 comprised the traceability from the manufacturer to the production by means of a GS1 DataMatrix on the primary and secondary packaging. The identification and batch numbers and the expiration date thereon are scanned and recorded. The second part of the project was dedicated to establishing the traceability from the production to the patient. Each recipient (AKH, St. Anna Children's Hospital, and the Hera Sanatorium) receives its own GLN (Global Location Number) and is thus already uniquely classified in the production phase. The serial number contains the medication number from the CATO system, thus making the cytostatic agent an individually identifiable product.
Electronic data reading for the preparation and administration of cytostatics is also used by the University of Geneva Hospital Center. More and more applications of this kind are being implemented in Europe, thus contributing to greater patient safety.
Global unique identification of subjects such as legal entities and locations and products such as consumer units, logistics units, etc. underlies the GS1 System of standards. In order for the identification to be automatically read, it is encoded with standard barcode symbols, using the eCom and GDSN standards and radio-frequency applications.
GS1 standards provide a harmonized approached which has proven itself worthy in time and in various sectors. Especially in healthcare harmonization in the identification of medicinal products is extremely important, because medicinal products go beyond the borders of the separate countries and it is very important thаt all participants along the supply chain use a unified universal approach, which also allows for traceability of the products. Barcode symbol encoding allows for automatic data processing, which helps minimize mistakes and create solutions allowing to put the 5 "R" rules into practice, namely: the right drug at the right time in the right dose for the right patient with the right documentation.
12.07.2012
Още новини:
-
05-07-2012

 1 USD =
1 USD =  1 GBP =
1 GBP =  1 CHF =
1 CHF =  ISO 9001:2015
ISO 9001:2015